
Microbial quality is one of the important factors that determine a cosmetic product to be safe for consumer use.
A number of measures can be taken to minimize microbial contamination before they are opened, for examples,
- Good Manufacturing Practices
- Suitable Preservative System
- Sealing or Packaging
- Storage Condition
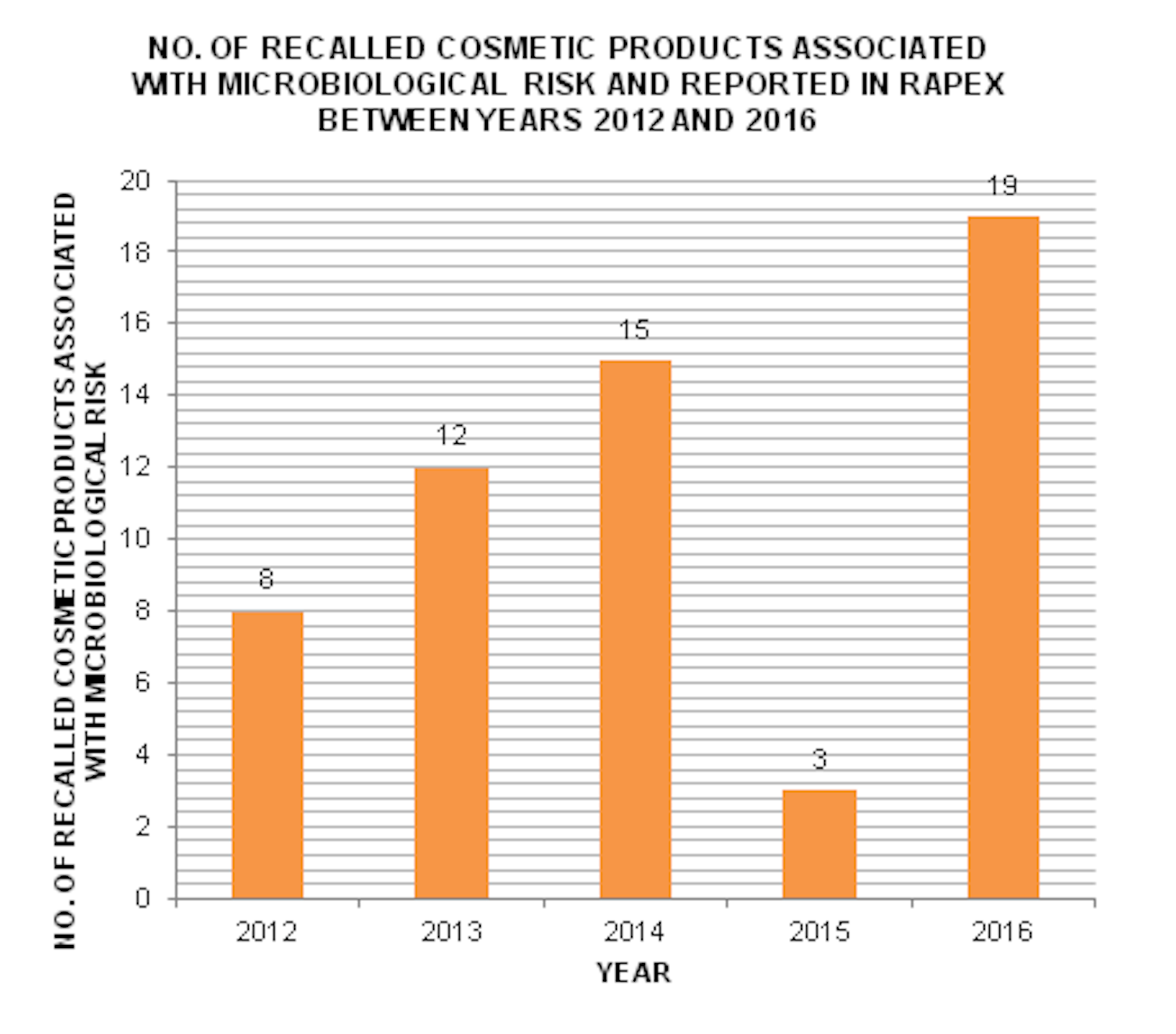
THE FEQUENCY OF COSMETIC PRODUCTS BEING RECALLED DUE TO MICROBIAL CONTAMINATION
There is an increasing trend of recalled cosmetic products which were found to be contaminated by microbes between 2012 and 2016.
In accordance to 2013/674/EU on Guidelines on Annex I to Regulation (EC) No 1223/2009 of the European Parliament and of the Council on cosmetic products, there are three kinds of cosmetic products,
(1) Low microbiological risk products
- Products with an alcohol content >20%
- Products based on organic solvents
- High/low-pH products
(2) Single-use products and products which cannot be opened
- Products for which the packaging allows dosing the product without it coming in contact with the air
(3) All other products
- Among all three kinds of cosmetic products, microbiological quality test is necessary except (1) low microbiological risk products.
QUANTITATIVE AND QUALITATIVE LIMIT IN MICROBIOLOGY
In Europe, the quantitative and qualitative limit in microbiology is given and provided in SCCS Notes of Guidance for the Testing of Cosmetic Ingredients and their Safety Evaluation, 9th revision (adopted on 29 September 2015, revised on 25 April 2016).
The cosmetic products are categorized into two groups, products specifically intended for children under three years of age, the eye area or the mucous membranes, and other products. In 9th revision of SCCS Notes of Guidance, the quantitative and qualitative limits are based on the European Standard EN ISO 17516:2014 Cosmetics – Microbiology – Microbiological Limits.
| Types of microorganisms | Products of Category 1 | Products of Category 2 |
Total Aerobic Mesophilic Microorganism (Bacteria plus yeast and mould) | ≤1X102 CFU per g or ml | ≤1X103 CFU per g or ml |
| Escherichia coli | Absence in 1g or 1ml | Absence in 1g or 1ml |
| Pseudomonas aeruginosa | Absence in 1g or 1ml | Absence in 1g in 1ml |
| Staphyloccocus aureus | Absence in 1g or 1ml | Absence in 1g or 1ml |
| Candida albicans | Absence in 1g or 1ml | Absence in 1g or 1ml |
Products of Category 1: products specifically intended for children under three years of age, the eye area or the mucous membranes
Product of Category 2: Other products (Except Products of Category 1)
If you would like to get this article in PDF format, please click here.
Should you have any inquiry, please contact
SGS HONG KONG LIMITED
Consumer and Retail
Cosmetics, Personal Care, and Household
Customer Service Team
t +852 2609 9611
e HK.CPCH.CSTeam@sgs.com
Units 303 & 305, 3/F, Building 22E,
Phase 3, Hong Kong Science Park,
Pak Shek Kok, New Territories, Hong Kong, China