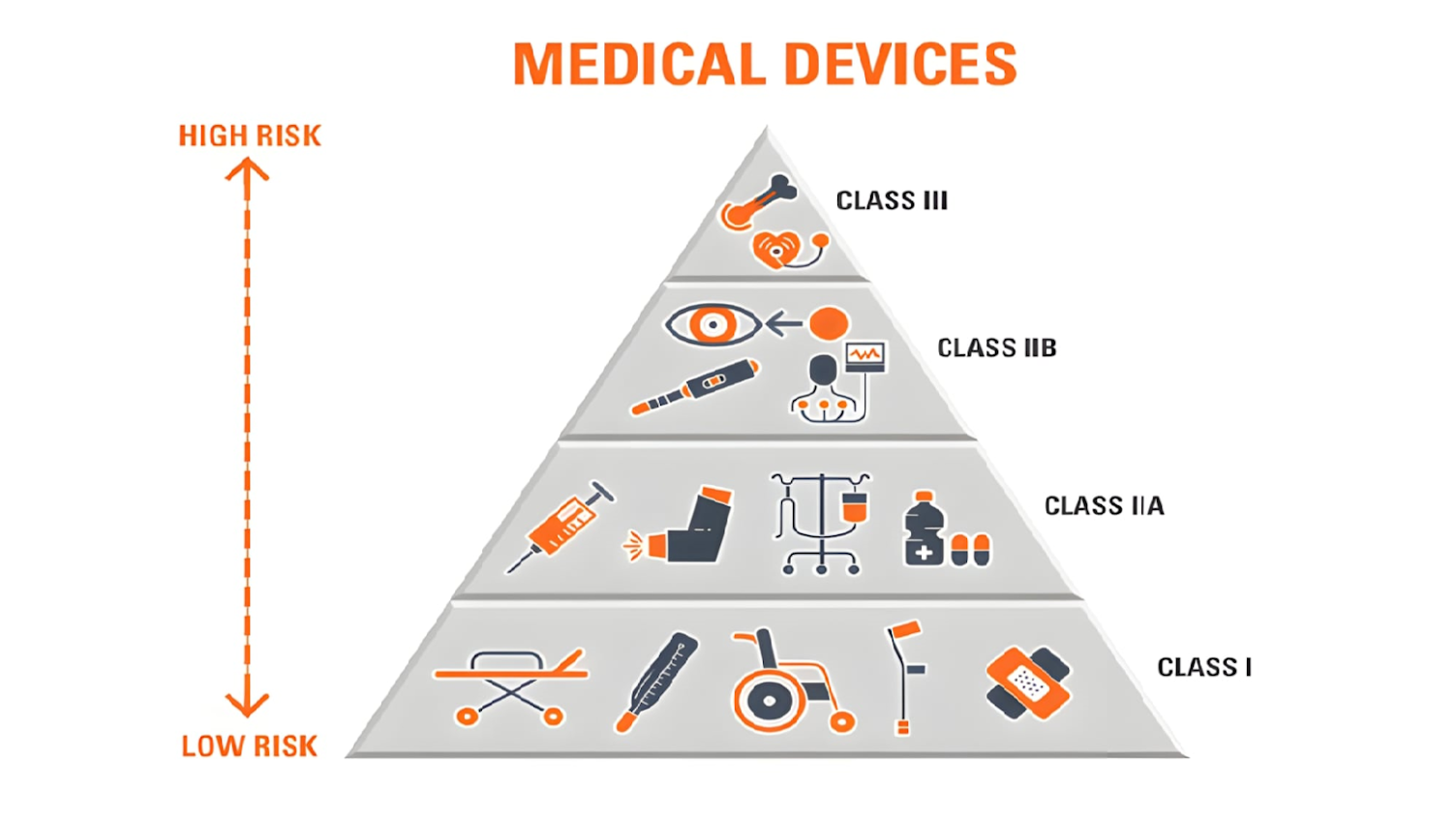
Many manufacturers struggle to properly classify their devices, which is not a trivial task. This is how we approach this question.
1. What type of organization do you represent regarding the medical device?
Confirm that you are the one required to determine the classification (in most jurisdictions: you are the legal manufacturer of the device). If you are a distributor, importer, contract manufacturer, etc., leave the classification to the legal manufacturer.
2. What is the intended use of the medical device?
Define the intended use and indications of your product, as these will determine the classification. Remember that the highest risk uses not excluded from the intended use will be used as the basis for classification, so explicitly exclude any use (e.g., in the central nervous system or central circulatory system) that is not required for your product; do leave the intended use, indications and contraindications vague or ambiguous. Note that some concepts (e.g., diagnostic medical devices) are interpreted differently in various jurisdictions (e.g., in Europe, devices providing information for clinician diagnostic decision making are also considered diagnostic devices, while in the US, this only includes devices that directly make clinical diagnosis).
3. Which markets are you targeting?
Define the target markets early on in the design and development process (don’t wait until the product is ready to see where you can market them), as this drives certain product specifications, e.g., GUI, labeling, IfU content and translations, necessary clinical evidence, etc.
4. Is it a medical device in the target markets?
Confirm that the product is defined and is regulated as a medical device in all the jurisdictions you plan to place them on the market (there can be differences, e.g., veterinary devices are medical devices in the US, personal hygiene devices like toothbrush are not medical device in Europe, etc.). See the regulatory definitions (as found in the original regulations and interpretation guidelines).
Pay special attention to borderline devices (e.g., medical devices that are/contain pharmaceutical drugs, machines, PPE, IVD elements, cosmetics or devices without clinical intended use).
If your device is not regulated as a medical device, refrain from fabricated intended uses just to justify the device being a medical device (e.g., with the intent to drive higher margin), regulatory approvals are not marketing tools. In this case just follow whatever other regulations apply to your device (e.g., LVD and EMC directives in Europe, etc.) or acknowledge (and appreciate) if none apply.
5. What are the relevant regulations that apply to your device?
Ultimately the classification is the responsibility of the legal manufacturer, other parties (including the notified body) may only have veto rights. When in doubt or disagreement, some regulatory authorities offer to verify your classification (e.g., US 513(g) or an EU NCA confirmation of classification), which will be treated as binding by the notified body.
400 Broadacres Drive,
Suite 200, 2nd Floor,
Bloomfield, New Jersey, 07003,
United States




