Integrated solutions that support your commitment to developing high quality biopharmaceutical and pharmaceutical drug products and medical devices.
Comprehensive solutions for drug product development
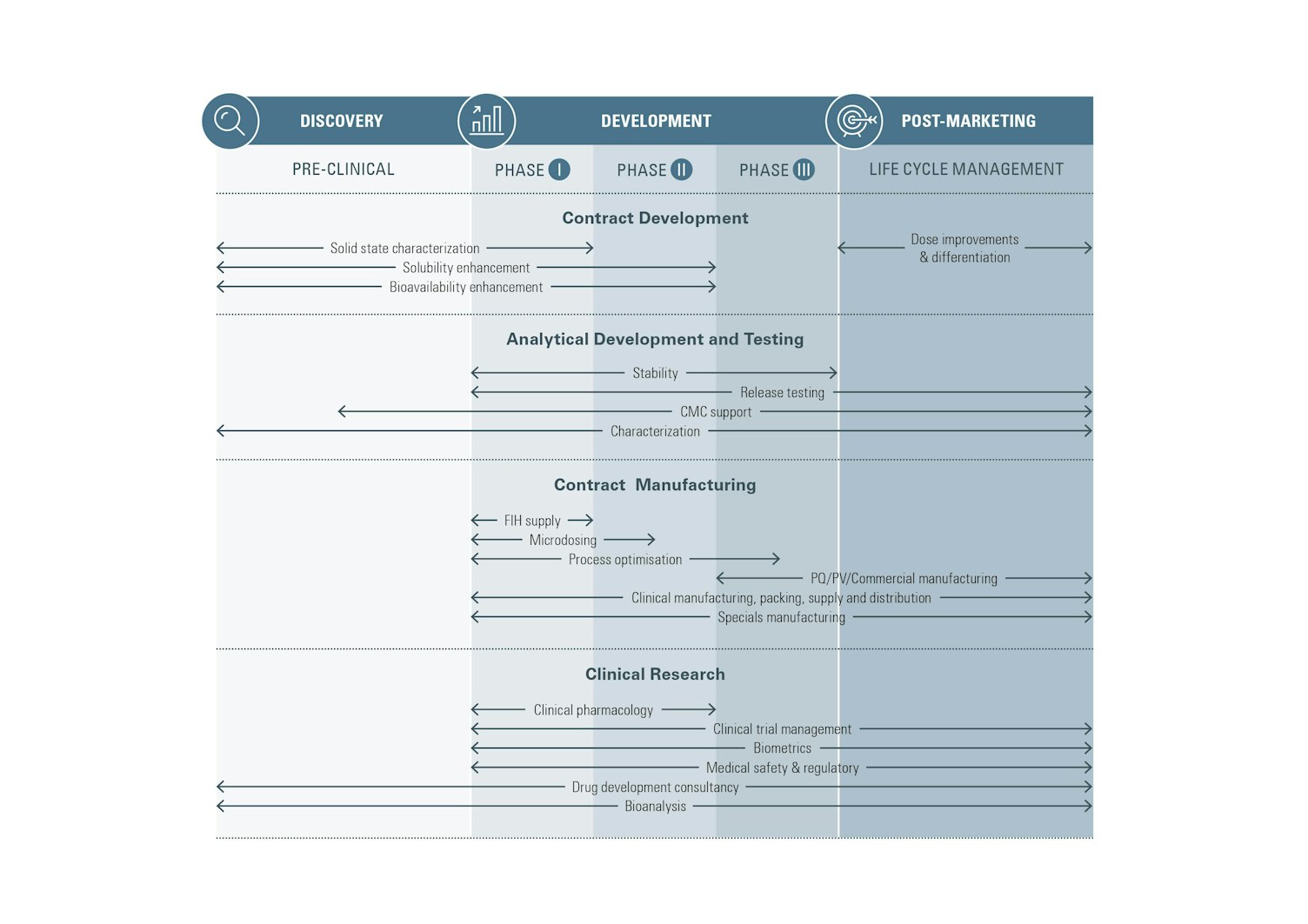
We provide best-in-class GMP/GLP analytical testing, formulation research and development, clinical manufacturing and clinical research services for the pharmaceutical, biopharmaceutical and medical device sectors. Our solutions match your biologics and small molecule needs and cover the complete life cycle – from exploratory development, testing, regulatory support, safety studies and clinical development, to commercial QC and post-market testing.
Utilizing a global network of laboratories and clinical trial facilities, our specialists deliver multifaceted, customer-centric programs at local and international levels. We help you to navigate your way to market while ensuring the optimization of drug exposure and product compliance with regulatory and industry standards and expectations.
With an increasing need to adopt agile, scalable resourcing models, we provide flexible sourcing models that let you focus on core competencies, improve overall performance and contain costs by leveraging our external management and quality assurance capabilities.

Looking for something specific?
Search within Pharma
616 Heathrow Drive,
Lincolnshire, Illinois, 60069,
United States