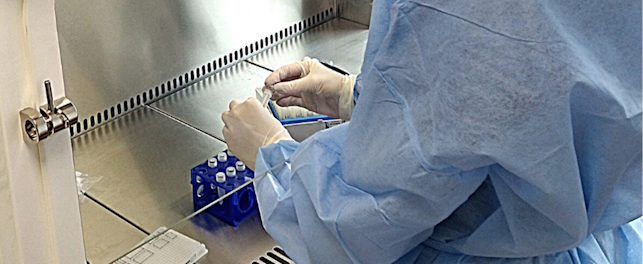
SGS, the world’s leading inspection, verification, testing and certification company, has invested in its Biosafety Center of Excellence in Glasgow – boosting its capacity to support scientists and manufacturers in the development and delivery of effective vaccines, cell and gene therapies and other biological medicines.
Pioneers in the development of the biosafety testing industry, this project has seen the construction of two new laboratories at SGS’s Glasgow Biosafety Center of Excellence. With new testing facilities, and an expanded specialist team, the ambition is to rapidly increase capacity for the specialized batch testing, a legal requirement before goods can be placed on the market.
Dr. Archie Lovatt, Scientific Operations Director, SGS said: “Our specialist team at the Biosafety Center of Excellence welcomes the far-reaching benefits this significant investment will bring. We are ambitious to do all we can to support the provision of vaccines to safely and effectively unlock the world from this pandemic.”
The construction of the two laboratories has been finalized and includes the existing building space into two separate GMP and BSL-2 laboratories with common changing facilities, dedicated air handling units and extract systems with safe change HEPA filtering. State-of-the-art equipment newly installed to support this includes specialized cell culture equipment for virus detection, ELISA technology and real-time PCR to ensure the safety of viral vaccines and other biological medicines. The work follows an earlier expansion of the Glasgow facility in 2019.
This expansion delivers increased capacity for the testing of cell banks for vaccines, gene and cell therapies, monoclonal antibodies and other recombinant protein based biological medicines, which includes a vaccine testing solution suitable for coronavirus and COVID-19. In addition, vaccine developers will gain easier access to specialized batch testing of their products, a legal requirement before goods can be placed on the market.
SGS Life Science Services
SGS leverages its digitalized network of life science laboratories, present in North America, Europe, and Asia-Pacific, to deliver harmonized testing solutions for analytical development, biologics characterization, biosafety and quality control, as well as clinical trial management to large pharmaceutical and biotechnology firms.
For further information, please contact:
Aurelia Resines
Global Marketing Manager
t: +41 22 739 91 11
About SGS
SGS is the world's leading inspection, verification, testing and certification company. SGS is recognized as the global benchmark for quality and integrity. With more than 89,000 employees, SGS operates a network of over 2,600 offices and laboratories around the world.