Understand the different types of UV radiation and the importance of employing a rigorous testing regime when developing and marketing sunscreens.
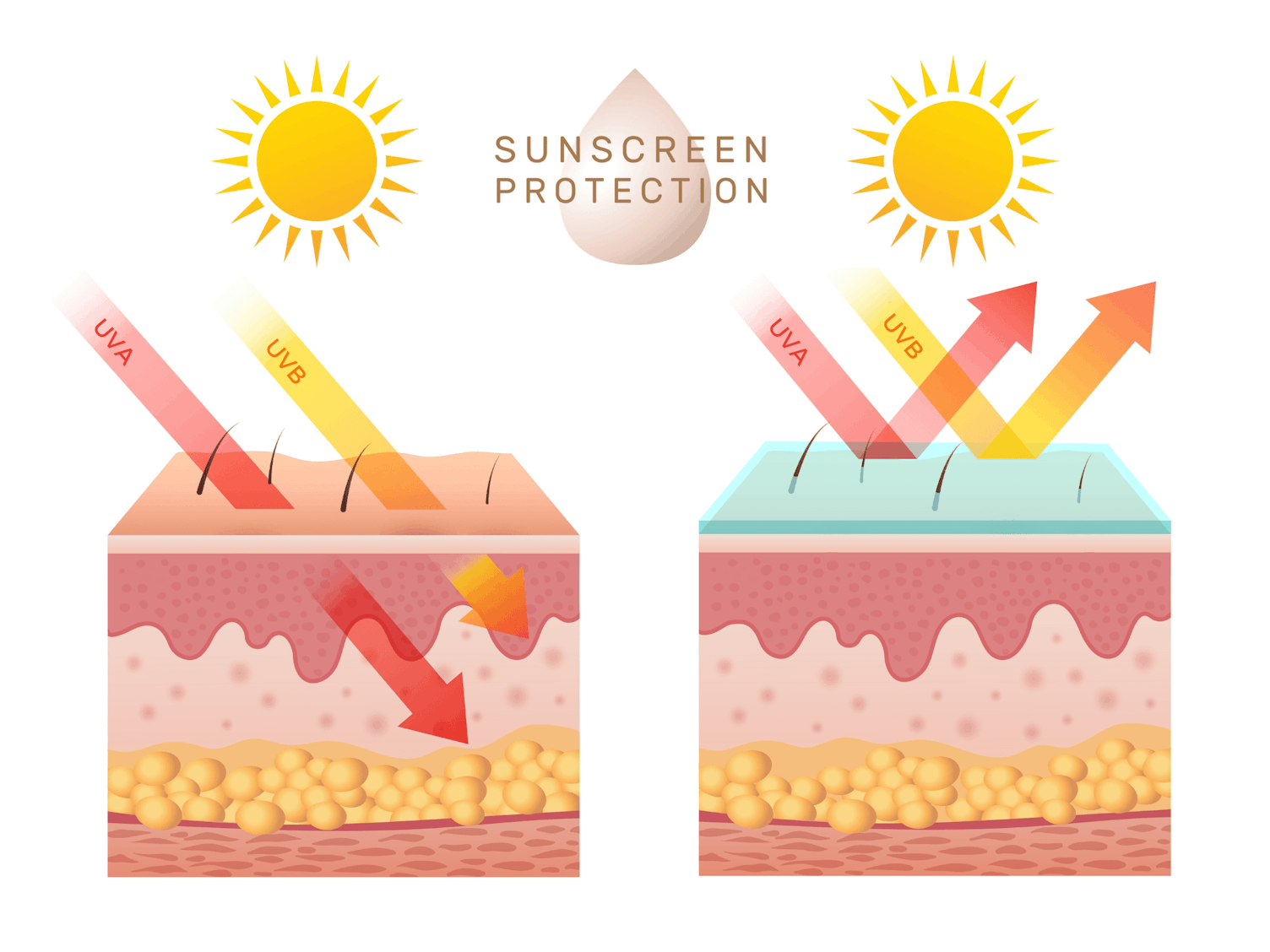
It is estimated one in five Americans will develop skin cancer in their lifetime. Skin serves as the body’s natural barrier, offering crucial protection against environmental factors. Throughout the day, it shields us from various stressors, including harmful ultraviolet (UV) radiation from the sun. Sunlight contains three types of UV rays – UVA, UVB and UVC. While UVC is absorbed in the ozone layer, both UVA and UVB reach the Earth’s surface, and our skin.
90% of the UV radiation that reaches the Earth is UVA. With a longer wavelength, UVA penetrates deeper into the skin, reaching the dermis. It can accelerate skin aging by damaging collagen and elastin, leading to wrinkles and sagging. It is also responsible for DNA damage and can trigger allergic reactions to sunlight.
UVB rays account for around 10% of the UV radiation that reaches the Earth. Due to a shorter wavelength, UVB is mostly absorbed by the epidermis, the skin’s outer layer. It is the primary cause of sunburn and can directly damage the DNA in skin cells, increasing the risk of skin cancer.
Safety, cosmetic appeal and efficacy
Sunscreen products must be safe and provide adequate protection from the sun. They should undergo multiple in vivo tests to ensure safety. Phototoxicity testing assesses whether the product causes skin irritation when exposed to sunlight – crucial for avoiding adverse skin reactions. Photoallergy testing examines the potential for allergic reactions triggered by sunlight exposure, ensuring the product is not a sensitizer. Non-comedogenicity testing determines whether the sunscreen will clog pores, which is essential for people who are prone to acne and other skin conditions. Finally, ocular testing evaluates a product’s safety if it comes into contact with the eyes, ensuring it does not cause irritation or harm.
SGS claim substantiation testing:
Safety:
- Photoallergy
- Phototoxicity
- Non-comedogenicity
- Ocular testing
Cosmetic acceptability:
- Home-Based SIU
- Consumer preference
Manufacturers also need to consider cosmetic acceptability. Sunscreen products must be tested for texture, scent and ease of application, as consumers generally prefer products that are easy to apply, non-greasy, and have a pleasant scent.
Efficacy is equally important. Sunscreen products need to be effective at protecting against harmful sun rays. Rigorous testing is necessary to measure the sun protection factor (SPF), which indicates the level of protection against UVB rays, and determine the level of UVA protection.
Essential tests and standards
Sun protection factor (SPF)
The US Food and Drug Administration (FDA), International Organization for Standardization (ISO) and Australian/New Zealand Standard (AS/NZS) have established tests to determine SPF, covering both dry and wet conditions.
- Dry SPF testing assesses a sunscreen's effectiveness in preventing sunburn reactions:
- FDA: designated as over-the-counter (OTC) drugs by the FDA’s 2011 sunscreen final rule, allowing them to be marketed without the need for a new drug application (NDA) or abbreviated new drug application (ANDA). However, updates proposed in 2019 aim to align sunscreen regulations with the latest scientific findings, particularly regarding the absorption of certain sunscreen ingredients into the body
- ISO 24444: outlines a method for determining SPF in vivo for sunscreen products. This standard applies to products intended for use on human skin that contain components which absorb, reflect or scatter ultraviolet (UV) rays. The SPF test outlined in ISO 24444 is currently utilized by over 70 nations, including: Australia, Canada, Chile, the European Union, India, Israel, Japan, Korea, Mexico, New Zealand, Russia, MERCOSUR countries, ASEAN countries, South Africa, Taiwan and the UK. In certain countries, alternative methods may also be recognized
- Wet SPF testing substantiates water resistance claims through various tests, including:
- FDA – 40 minutes/80 minutes
- ISO 16217:2020 – 40 minutes/80 minutes
- ISO 18861.2020 – 40 minutes/80 minutes
- AS/NZS 2604:2021 – 40 minutes/2 hours/4 hours
UVA protection factor (UVA-PF)
In vivo testing for UVA is conducted through persistent pigment darkening and follows regulatory guidelines set by ISO, specifically outlined in ISO 24442:2022.
In-vitro testing
- Photostability: assesses how well sunscreen holds up under sunlight. The effectiveness of SPF, UVA protection and critical wavelength are checked across the UV spectrum. A sunscreen's stability is assessed by measuring its SPF before and after exposure to UV light. Test options include hybrid diffuse reflectance spectroscopy (HDRS) and ISO 24443:2021
- Broad spectrum protection ensures shielding against both UVA and UVB radiation. To substantiate a broad-spectrum claim, a sunscreen must filter against the majority (90%) of the UVB/A spectrum, spanning from at least 290nm to 370nm. Test standards include:
- ISO 24443:2012 and ISO 2443:2021
- FDA OTC Monograph M020
- Ecotoxicity: in partnership with REEFTOX, the acute ecotoxicity of active ingredients and cosmetic formulations on coral reef can be assessed. Reef-friendly claims can be tested using:
- ReefTox 12: panel of 12 coral species
- ReefTox 50: panel of 50 coral species
- Physical and chemical properties: our solutions evaluate physical characteristics such as pH, viscosity, density, flash point and mass, and identify and quantify active substances, particularly UV filters. Advanced analytical techniques include:
- High-performance liquid chromatography (HPLC) and ultra-performance liquid chromatography (UPLC), equipped with detectors such as UV, PDA, FLD, RI, Corona and ELSD
- Gas chromatography (GC) with Headspace (HS) capabilities, featuring detectors including TCD, FID and MS
SGS joins consortium for alternative testing methodologies
We have joined an international consortium to assess and validate innovative methods for testing sunscreens that do not rely on visible biological reactions. The HDRS test method, detailed in draft ISO 23698, is a key tool used to evaluate SPF, UVA-PF and critical wavelength protection in sunscreen products. This method measures the spectral absorbance properties of sunscreen applied to the skin, providing essential data to estimate protection against sunburn and UVA radiation. It offers an alternative to traditional methods, such as ISO 24442, ISO 24443 and ISO 24444, for products designed to interact with UV rays and intended for skin contact.
About SGS
We are SGS – the world’s leading testing, inspection and certification company. We are recognized as the global benchmark for sustainability, quality and integrity. Our 99,600 employees operate a network of 2,600 offices and laboratories around the world.



