Introduction
As a medical device Notified Body (NB 1639) we are designated to certify all types of medical devices, including those without an intended medical purpose. Once certified with us, you will be entitled to affix the CE 1639 mark to your medical devices and to place them on the European Union market.
We can also provide quality management certificates to distributors or importers carrying out any activity mentioned in points (a) and (b) of MDR Article 16(2), subject to an application and audit procedure.
The exact scope of our designation (SGS Belgium NV (NB 1639)) can be found on the official Nando database: New Approach Notified and Designated Organisations
If you have any questions regarding the certification process, or if you are uncertain which SGS facility can support you through your certification journey, please contact us.
Information for new clients
In order to meet your needs, we provide our services globally via a network of affiliates, referred to as SGS Delivering Offices. Your local SGS Delivering Office will serve as your primary point of contact throughout your certification process.
The conditions established in the following documents apply to your CE certification:
- SGS Code of Practice
- SGS General Conditions for Certification Services
- Regulations Governing the Use of SGS Certification Marks
Contact your SGS Delivering Office to obtain more information about the certification process.
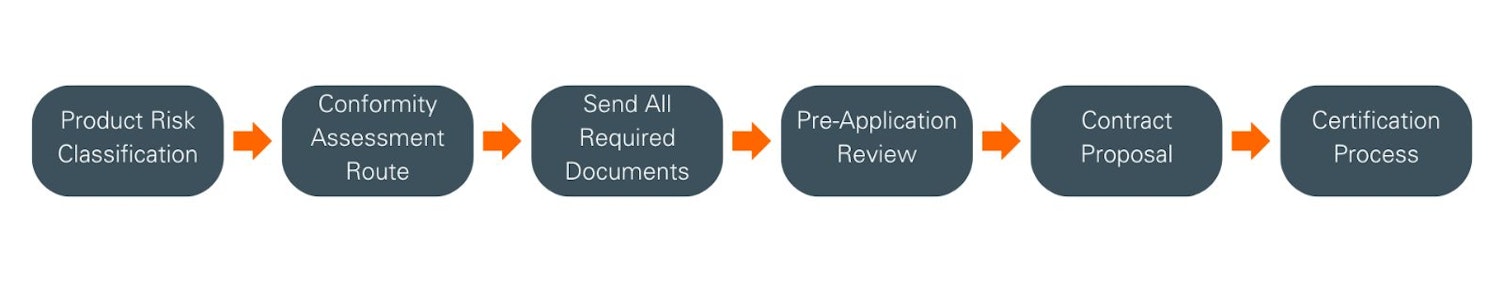
Product risk classification & conformity assessment route
Your first step is to determine your product(s) classification, in accordance with the rules defined in Annex VIII of the MDR. For clarification and further guidance, please refer to MDCG 2021-24.
Next, you must decide which type of conformity assessment path you wish to apply:
- Conformity assessment based on the Quality Management System (QMS) and Technical Documentation Assessment (TDA), as per Annex IX of the MDR
- Conformity assessment based on Product Conformity Verification (Production Quality Assurance), as per Annex XI Part A of the MDR
For devices that are “medicinal” and governed by Directive 2001/83/EC, according to Article 8 of the MDR, we can propose an assessment, according to MDR Article 117, to provide a Notified Body opinion on compliance with GSPR for the medical device part of a drug-device combination.
Please consult Your Certification Process Explained – MDR for guidance on the conformity assessment process applicable to your device(s) and the certification we may offer.
Lodging your application
Once you have determined the product’s risk classification and decided on a conformity assessment route, the certification journey can begin. To initiate this process, please contact your local SGS Delivering Office, which will provide you with all the application documents that must be completed and returned. Please be advised that your local SGS Delivering Office remains fully available to offer comprehensive support throughout each stage of your certification.
Please note that your application, technical documentation and any subsequent correspondence must be submitted in English. However, we understand that your QMS may be in either your local language or English.
Voluntary change of notified body
If you possess certificates with another notified body, you may decide to undertake a voluntary change of notified body in accordance with Article 58 of Medical Device Regulation (EU) 2017/745 and transfer certification to SGS NB 1639 at any point within your certification cycle. Please be advised that a voluntary change of notified body can only occur while your current certificates are valid. For more information regarding voluntary change of notified body and applicable process, please consult Your Certification Process Explained – MDR.
If you are uncertain whether you meet the criteria, contact us to discuss the available options for certification with SGS NB 1639.
Fees
A list of standard fees for conformity assessment activities under MDR (2017/745) is available in the Important documents section.
Please contact your local SGS Delivering Office to discuss a price estimate tailored to your device portfolio.
Other medical device certification services offered by SGS
The global regulatory landscape for medical device products and services is complex, and many of our clients have a global reach. We offer a broad portfolio of certification and accreditation services covering various national and international requirements. Whether your organization currently has a global reach, or you are planning to enter additional markets in the future, we will be able to support you in your certification journey with a service tailored to your needs.
Please consult your local SGS Delivering Office representative to receive a further guidance on how our range of offerings can help your products achieve a global reach. Our certification services include:
For more information, visit the SGS Medical Devices Regulatory Compliance web page.