Orthopedic devices cover a broad spectrum, ranging from implants and instruments to rehabilitation tools. To ensure that the EU Notified Body or UK Approved Body is qualified to review a specific device, devices are categorized and grouped based on the intended use or technologies employed in the device. For the convenience of the manufacturer, we use a single coding system for both the EU and the UK. The devices are allocated codes based on the publicly accessible EU MDR code list from commission implementation regulation (EU) 2017/2185 of Nov 23, 2017, and guidance from the Medical Device Coordination Group Document MDCG 2019-14.
For orthopedic devices, these codes may include:
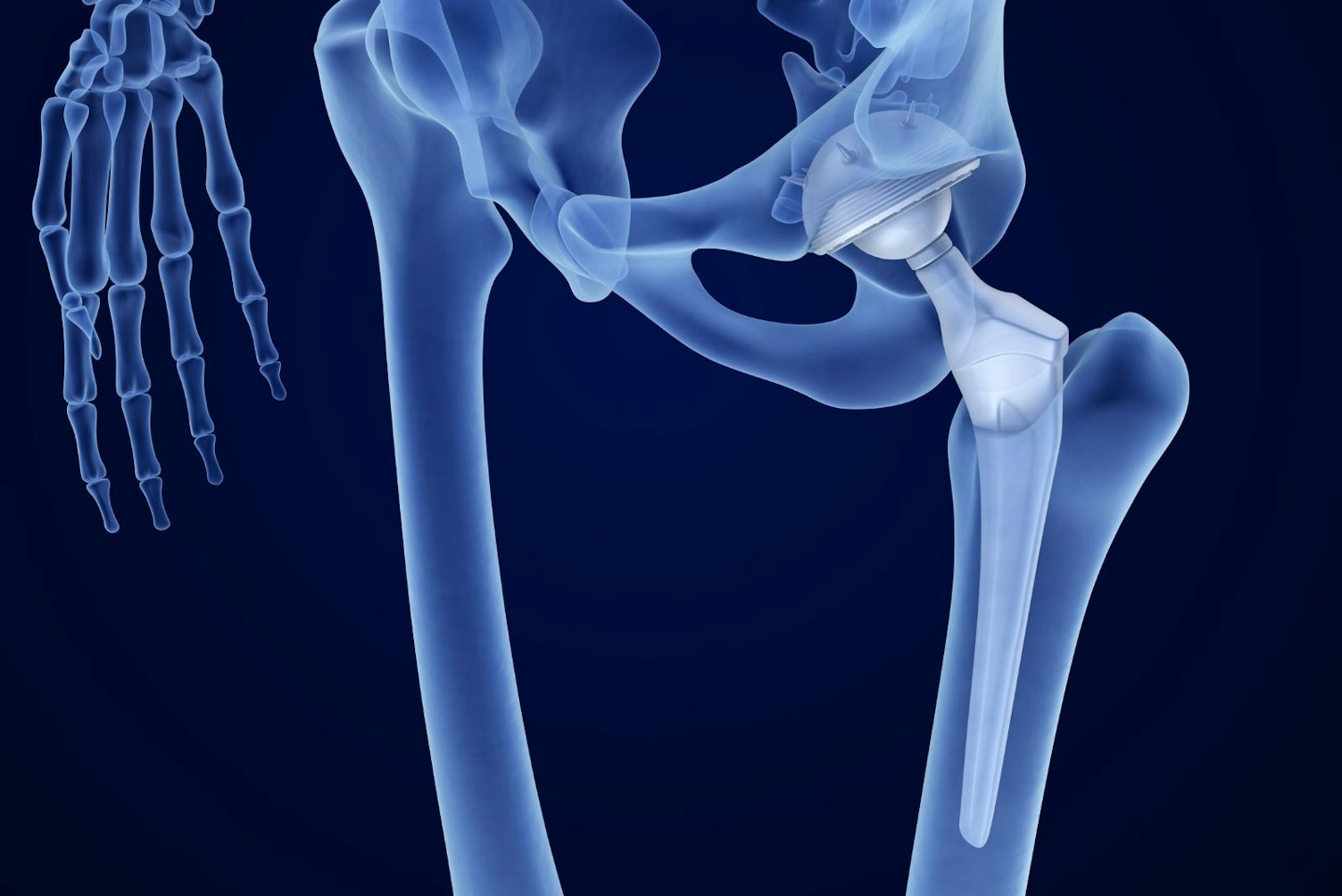
- MDN 1102: non-active osteo- and orthopedic implants. These include:
- Joint (hip, knee shoulder, ankle) and spinal disc replacements
- Bone graft substitutes for orthopedic and maxillofacial use
- Sutures and suture anchors
- Orthopedic surgery staples
- Spacers
- Ligament reconstruction products
- Osteosynthesis devices, such as orthopedic nails, screws, and plates
- MDN 1205: non-active, non-implantable orthopedic and rehabilitation devices. It includes devices like orthoses, crutches, and wheelchairs, which are crucial for supporting patient mobility
- MDN 1208: non-active, non-implantable instruments such as forceps, clamps, scalpels, reamer, drill bits, mallets, bone spoons, curettes, retractors, elevators, burs, spatulas, and patient-specific instrumentation (PSI). It also covers non-active devices used in conjunction with arthroscopic equipment, such as arthroscopy trocars
To support efficient conformity assessments, devices are also grouped using the European Medical Device Nomenclature (EMDN) for the EU, or the Global Medical Device Nomenclature (GMDN) in the UK, to identify and categorize orthopedic devices that can be assessed together.